Chemistry (313)
Tutor Marked Assignment
Q 1. (a) Classify the elements of group 14, 15 and 16 into metals, non-metals and metalloids.
Ans-
The elements of Group 14 are classified as:
- Metals: Tin (Sn) and Lead (Pb)
- Metalloids: Carbon (C), Silicon (Si) and Germanium (Ge)
- Non-metal: None
The elements of Group 15 are classified as:
- Non-metals: Nitrogen (N), Phosphorus (P), Arsenic (As),
- Antimony (Sb) and Bismuth (BI)
- The elements of Group 16 are classified as:
- Non-metals: Oxygen (OsanSelenium (Se), Tellurium (Te) and Polonium (Po)

Watch Full TMA:https://youtu.be/1dECc3d9Eas
Contact For TMA PDF: 84487 31058 (WhatsApp)
Hindi PDF Link: https://imojo.in/xsPOnr
Q 2. (a) How do metallic and ionic substances differ in conducting electricity?
Ans-
- Metallic substances- Metallic substances conduct electricity due to the free movement of electrons. These electrons can move throughout the metal, carrying electric charge.
- ionic substances- lonic substances conduct electricity when dissolved in water or melted. The ions in the solution can move freely, carrying electric charge. However, ionic substances do not conduct electricity in their solid state due to the fixed position of ions in the crystal lattice.
Q 3. (b) What must be the value of (Ea) if the rate constant for a reaction is doubled when the temperature increases from 300K to 310K?
Ans- The activation energy (Ea) can be estimated using the Arrhenius equation. Given that the rate constant doubles with a 10K increase from 300K to 310K, we apply the equation:
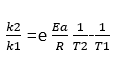
Solving for Ea, it is approximately 53.6 kJ/mol.
Q 4. (b) Calculate the equilibrium constant for the reaction A(g) + B(g) ⇒ C(g) + D(g) If at equilibrium 1 mol of A, 0.5 mole of B, 3.0 mole of C and 10 mol of D are present in a one litre vessel.
Ans-
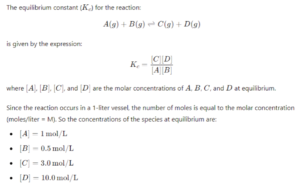
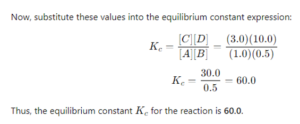
Q 5. (a) Describe the underlying principle of each of the following metal refining methods:
(i) Electrolytic refining of metals
(ii) Vapour phase refining of metals
Ans- Here are the underlying principles for each metal refining method:
(i) Electrolytic Refining of Metals
Principle: Electrolytic refining is based on the principle of electrolysis, where impure metal is purified by using an electrolytic cell. The impure metal acts as the anode, and a thin strip of pure metal is used as the cathode. The electrolyte is usually a solution of a salt of the metal to be refined.
Process:
When an electric current pass through the electrolyte, metal atoms from the anode dissolve into the solution and are deposited on the cathode as pure metal. Impurities either fall to the bottom as anode mud or remain in the electrolyte, depending on their nature.
For example, in the electrolytic refining of copper, the impure copper anode dissolves into the copper sulphate solution, and pure copper is deposited on the cathode, while impurities like gold, silver, and platinum settle as anode mud.
(ii) Vapour Phase Refining of Metals
Principle: Vapour phase refining is based on the principle that certain metals can be converted into their volatile compounds and then decomposed to obtain pure metal. This method exploits the ability of metals to form volatile compounds with specific reagents, which can then be easily separated and decomposed.
Two Common Examples:
Mond Process for Nickel Refining:
- Nickel is heated in the presence of carbon monoxide (CO) at around 330–350 K to form nickel carbonyl (Ni (CO)4) a volatile compound.
- This compound is then decomposed at higher temperatures (around 450–470 K), resulting in pure nickel.
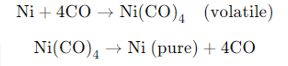
Van Arkel Process for Zirconium or Titanium Refining:
- Impure zirconium or titanium is reacted with iodine to form volatile metal iodides (e.g., ZrI₄).
- These iodides are then decomposed on a hot filament (usually tungsten) to give pure metal and iodine.
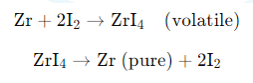
- Thus, vapour phase refining allows the separation of metals based on the volatility of their compounds, followed by thermal decomposition to yield pure metal.
Q 6. (b) Chemistry is deeply embedded in our daily experiences. Answer the following questions regarding the presence and impact of chemistry in your everyday life.
(i) How do you perceive the presence of chemistry in your daily routines or activities? Can you identify any specific examples where chemistry plays a significant role? (e.g., food preparation, cleaning products, personal care items, etc.)
(ii) Have you ever considered the chemical composition or reactions behind common household items or products you use? Please provide examples and elaborate on your understanding.
(iii) In what ways does chemistry influence the food you consume or prepare? How do chemical reactions impact cooking or food preservation techniques?
(iv) Can you identify any environmental issues related to chemical usage in everyday life? How do you think these issues could be mitigated or improved?
(v) How does chemistry contribute to advancements in medicine and healthcare? Can you discuss any specific pharmaceutical products or medical procedures that showcase the role of chemistry in improving human health?
(vi) What role does chemistry play in personal hygiene products or cosmetics that you use? Are you aware of any chemical risks associated with these products?
Ans-
(i) Chemistry in Daily Routines or Activities Chemistry is all around us, and I often notice its influence in activities such as:
- Food Preparation: Cooking involves chemical changes, such as the Maillard reaction (browning of food) and caramelization of sugars. Boiling, frying, or baking all involve heat-induced reactions that alter the structure and flavour of food.
- Cleaning Products: Soaps, detergents, and disinfectants use chemistry to remove dirt, oils, and bacteria. Surfactants in soap reduce surface tension, allowing water to better clean surfaces.
- Personal Care Products: Shampoos, deodorants, and toothpaste contain chemicals designed for specific functions, such as cleaning, killing germs, or moisturizing.
(ii) Chemical Composition of Household Items Yes, I often think about the chemical composition of household products:
- Baking Soda (Sodium Bicarbonate): I use it in baking where it acts as a leavening agent, releasing carbon dioxide gas when it reacts with acids. It is also used for cleaning, neutralizing odours, and even for skincare.
- Vinegar (Acetic Acid): It’s useful for cooking and cleaning. The acidic nature of vinegar can break down limescale and dissolve grime.
- Bleach (Sodium Hypochlorite): Used for disinfecting, bleach works by releasing oxygen molecules that break down the stains or kill germs, though it needs careful handling due to its corrosive nature.
(iii) Chemistry in Food and Cooking
Chemistry greatly influences food in terms of flavour, texture, and safety:
- Cooking: Heat causes chemical reactions like protein denaturation in eggs or meat, and starch gelatinization in pasta or rice. These reactions change the structure of the food, making it more digestible and flavourful.
- Food Preservation: Techniques like canning, freezing, and drying all use chemical principles to prevent spoilage. Salt and sugar act as preservatives by drawing water out of food, inhibiting bacterial growth.
- Fermentation: The process used in making yogurt, bread, or beer relies on the conversion of sugars into alcohol or acids by bacteria and yeast.
(iv) Environmental Issues Related to Chemical Usage Many environmental issues are linked to chemical usage in daily life:
- Plastic Waste: Plastics are made from non-biodegradable polymers, leading to pollution in oceans and landfills. Reducing plastic use and recycling are essential steps to mitigate this problem.
- Chemical Pesticides: These chemicals can harm wildlife, contaminate water sources, and contribute to soil degradation. Switching to organic farming or using less harmful biopesticides could reduce environmental harm.
- Air Pollution: Many household products, like aerosols or cleaning agents, release volatile organic compounds (VOCs) that contribute to air pollution and respiratory problems. Using eco-friendly products with fewer VOCs can help mitigate these effects.
(v) Chemistry in Medicine and Healthcare
Chemistry plays a fundamental role in developing life-saving drugs and medical treatments:
- Pharmaceuticals: Medications like aspirin (acetylsalicylic acid) relieve pain and reduce inflammation by chemically inhibiting enzymes. Antibiotics like penicillin kill bacteria by interfering with their cell wall synthesis.
- Vaccines: They are made using chemistry to formulate safe and effective antigens that trigger the body’s immune response.
- Medical Imaging and Diagnosis: Techniques like MRI and CT scans involve chemical principles, using contrast agents to enhance images and diagnose conditions.
(vi) Chemistry in Personal Hygiene and Cosmetics
Personal hygiene products and cosmetics are made of chemicals that perform specific functions:
- Shampoo: Contains surfactants like sodium lauryl sulfate that help break down oils and dirt from hair.
- Sunscreen: Uses chemicals like zinc oxide or avobenzone to block harmful UV rays and protect the skin from sun damage.
- Cosmetics: Lipsticks, foundations, and creams contain pigments, emollients, and preservatives, which make them more effective, though some may contain chemicals like parabens that are linked to health risks.
- Awareness of Chemical Risks: I am mindful of potential risks associated with these products, such as skin irritation or long-term exposure to harmful chemicals like formaldehyde or phthalates. Choosing products labelled as paraben-free or using natural alternatives can reduce exposure to these risks.
Conclusion
In conclusion, chemistry is deeply intertwined with everyday life, influencing our health, environment, and lifestyle. Understanding its role helps us make more informed decisions.